Invited talk
Steric Crowding in Covalent Organic Frameworks
Aurelio Mateo-Alonso
POLYMAT, University of the Basque Country, Avenida de Tolosa 72, 20018 San Sebastian
Ikerbasque, Basque Foundation for Science, Bilbao
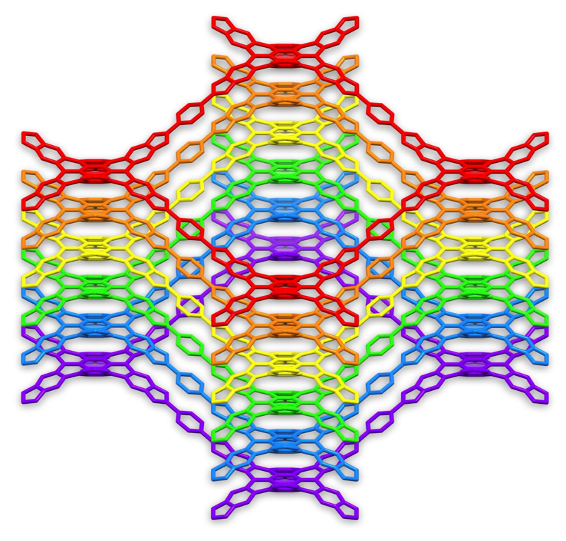
Polycyclic aromatic hydrocarbons can adopt a broad range of non-planar conformations that challenge the perception of aromatic systems as rigid and flat structures. Such distorted structures are the result of the steric strain induced by overcrowding or congestion in key positions of the aromatic core. Distorted aromatics have shown enhanced solubility and unique optoelectronic and chiroptical properties as an effect of their distorted molecular structure. Our group has introduced the use of distorted aromatics in framework materials.1-5 This merge offers new possibilities in the design monomers with symmetries that deviate from standard graphitic geometries, and in turn, in the design of 2D and 3D covalent organic frameworks with unprecedented architectures and properties. Furthermore, the incorporation of monomers with sterically demanding substituents can disrupt the interactions between layers, affecting crystallinity, layer arrangement, topology, interlayer distances, or even producing selfexfoliation. The most recent advances of these distorted framework materials including synthetic routes, optoelectronic properties, self-organising properties, and potential applications will be discussed.
References
1. A. B. Marco, D. Cortizo-Lacalle, I. Perez-Miqueo, G. Valenti, A. Boni, J. Plas, S. De Feyter, F. Paolucci, M. Montes, A. Khlovystov, M. Melle-Franco and A. Mateo-Alonso Angew. Chem. Int. Ed., 2017, 56, 6946.
2. M. Martínez-Abadía, C.T. Stoppiello, K. Strutynski, B. Lerma-Berlanga, C. Martí-Gastaldo, A. Saeki, M. Melle-Franco, A.N. Khlobystov and A. Mateo-Alonso J. Am. Chem. Soc. 2019, 141, 14403.
3. M. Martínez-Abadía, K. Strutynski, B. Lerma-Berlanga, C. T. Stoppiello, A. N. Khlobystov, C. MartíGastaldo, A. Saeki, M. Melle-Franco, and A. Mateo-Alonso Angew. Chem. Int. Ed., 2021, 60, 9941.
4. A. Riaño, K. Strutyński, M. Liu, C. T. Stoppiello, B. Lerma-Berlanga, A. Saeki, C. Martí-Gastaldo, A. N. Khlobystov, G. Valenti, F. Paolucci, M. Melle-Franco, and A. Mateo-Alonso, Angew. Chem. Int. Ed., 2022, 61, e202113657.
5. De Bolòs, M. Martínez-Abadía, F. Hernández-Culebras, A. Haymaker, K. Swain, K. Strutyński, B. Weare, J. Castells-Gil, N. M. Padial, C. Martí-Gastaldo, A. N. Khlobystov, A. Saeki, M. Melle-Franco, B. L. Nannenga and A. Mateo-Alonso, J. Am. Chem. Soc., 2022, 144, 15443.